
Jun 5, 2023 — Registrational study designed to support BLA submission of first-in-class biotherapeutic.
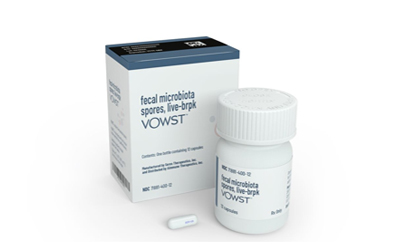
Apr 26, 2023 — The drug is the first microbiota-based pill to win U.S. approval. Like a rival therapy approved in December, it’s for difficult-to-treat C. diff infections.

Oct 18, 2022 — Positive results include clinically meaningful Phe reductions, with a 60% response rate and 42% reduction in plasma Phe among responders across the study population

Nov 10, 2021 — Collaboration will leverage Novome’s GEMMs platform for colonizing the human gut with therapeutically engineered bacteria
和度生物医药(上海)有限公司 沪ICP备2021004953号-1